Enhance Software Reliability with IV&V Testing
Dulles IV&V Testing Services

-
Mitigate Risks Early
Identify and address potential issues early in the development cycle, reducing the risk of costly errors and ensuring your software performs reliably and meets all requirements.
-
Enhance Compliance
Ensure your software adheres to industry standards and regulatory requirements, minimizing the risk of non-compliance and protecting your organization from potential penalties.
-
Boost Confidence
By providing an independent assessment, IV&V services increase confidence in your software's quality and performance, ensuring it functions as intended and meets user expectations.
Ensure Software Quality with IV&V Services
Our Process
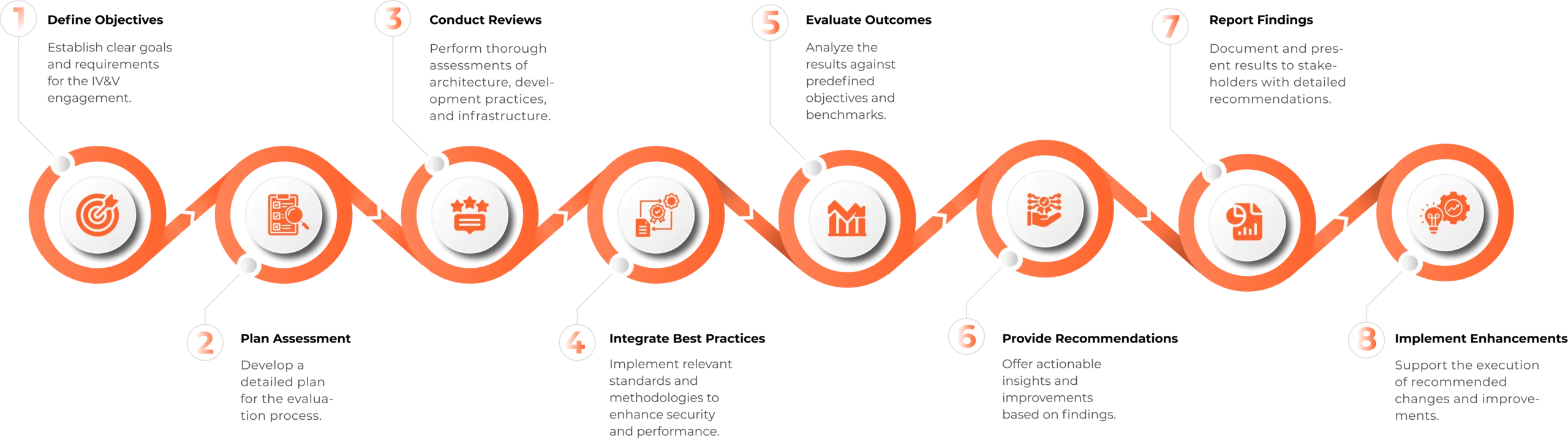

Dulles Impact

Leveraging IV&V for Optimal System Performance
Leveraging IV&V for Optimal System Performance
Achieving project excellence requires more than just development; it demands a thorough and balanced evaluation. Independent Validation and Verification (IV&V) services provide exactly this, ensuring that systems meet their intended requirements and deliver outstanding performance.
IV&V encompasses a comprehensive assessment of system architecture, infrastructure, application development practices, adequate testing, deliverables and overall program management processes. These detailed reviews help identify potential risks early, allowing proactive resolution and preventing any negative impact on project success.
The approach integrates industry best practices, including advanced security measures and performance optimizations, to enhance system reliability and effectiveness. Detailed, actionable reports and recommendations are provided, enabling informed decision-making and driving continuous improvement.
Our client:
The mission of the Center for Devices and Radiological Health (CDRH) is to protect and promote public health. The CDRH assures that patients and providers have timely andcontinued access to safe, effective, and high-quality medical devices and safe radiation-emitting products. CDRH facilitates medical device innovation by providing industry with efficient regulatory pathways, and assuring consumer confidence in devices marketed in the U.S.
CDRH is implementing an agency-wide significant Digital Transformation [DT Program] initiative, designed to enhance IT services, streamline operations, and ultimately improve public health outcomes. DullesSystems provided a robust Independent Verification and Validation (IV&V) process, crucial for ensuring the program’s success and effectiveness.
By focusing on increasing the reliability, timeliness, and availability of data, the program transforms this critical asset into a powerful tool for informed decision-making. Additionally, implementing Master Data Management (MDM) establishes a single, accurate version of data, promoting consistency across the organization. The initiative also seeks to optimize IT service delivery, achieving greater efficiency and cost-effectiveness.
Challenges Faced by the Client
- Agile Delivery Process: Misaligned goals, Insufficient stakeholder engagement, inadequate estimation and dependency identification.
- User Experience: System improvements for more intuitive and efficient use by internal staff and external stakeholders.
- Test coverage: Lack of quality control in test coverage, mapping between requirements and test cases results in inadequate requirement traceability matrix generation.
- Program management- Enhancing compliance in quality, cost, scope and time for better earned value management[EVM].
- Strengthened IT Governance: Required better oversight and data-driven decision-making to align IT investments with strategic goals.

- Data Reliability: Reliability, timeliness, and availability of critical data for informed decision-making for Leadership .
- Unified Data Management: Accurate and consistent data Single Version of Truth [SVT] across the organization.
- Optimized IT Service Delivery: Needed to enhance service delivery while reducing costs and improving overall operational efficiency.
DullesSystems' Solution
- Independent Performance Evaluation: Conducted rigorous assessments of the DT Services contractor’s work to ensure alignment with program goals and high-quality standards.
- Objective Feedback: Provided unbiased, actionable insights to guide CDRH leadership in making informed decisions and addressing potential issues.
- Regulatory Compliance: Validated that all products and services adhered to FDA standards and best practices, ensuring accuracy, completeness, and effectiveness.
- Validation of Deliverables: Ensured that all deliverables meet defined requirements and fulfilled the program’s objectives effectively.
- Program Level Monitoring– Review Project charter, objectives, scope, Project initiation to closure deliverable to provide an overall program Health check.
How DullesSystems Added Value
Please refer to the following diagram:

- Agile Process Tune up: Thoroughly discovered and summarized the reasons on the retrospective, and implemented improvements to the next sprint and thereby enhanced agile executions.
- Enhanced Confidence: Reduced risks and errors by identifying and resolving issues early in the development process. Boosted confidence in product quality and delivery schedules, facilitating smoother project execution.
- Streamlined Implementations: Optimized system deployments and integrations, improving performance and addressing operational challenges efficiently.
- Improved User Acceptance: Ensured solutions meet user needs, leading to greater satisfaction and successful realization of program benefits.
- Exceeding Expectations: Delivered transformative results that not only met but exceeded the expectations of the CDRH, setting a new benchmark for digital excellence.
















